About the Summit
Welcome to the 8th Microbiome Movement - Drug Development Summit Europe
As Europe’s premiere microbiome conference tightly focused on the development of microbiome-based therapeutics, this is was your prime opportunity to expand your microbiome-based drug development capabilities and business potential as we aim to deliver clinical results and ensure therapeutic microbiome approval in Europe.
No matter what stage of development you were focusing on, this was your one-stop shop for microbiome-based drug development from early discovery to late-phase manufacturing!
What You Missed In 2024:
- Highlighting early-stage organisations and their novel technologies in our brand-new technology showcase where we welcome Centre for Genomic Regulation, Recolony and Synflora to give quick fire presentations to showcase their work.
- Discovering our new enabling technologies track to learn more about the exciting artificial intelligence, integrated -omics analysis and improved manufacturing capabilities among the other technologies that are accelerating microbiome-based drug development.
- Strengthening your regulatory understanding and enhance decision-making by uncovering global regulations to address updated SoHo frameworks, explore considerations for engineered microbes and consolidate agencies interactions
BRAND NEW C-LEVEL MICROBIOME TRAILBLAZERS JOINED US IN 2024:







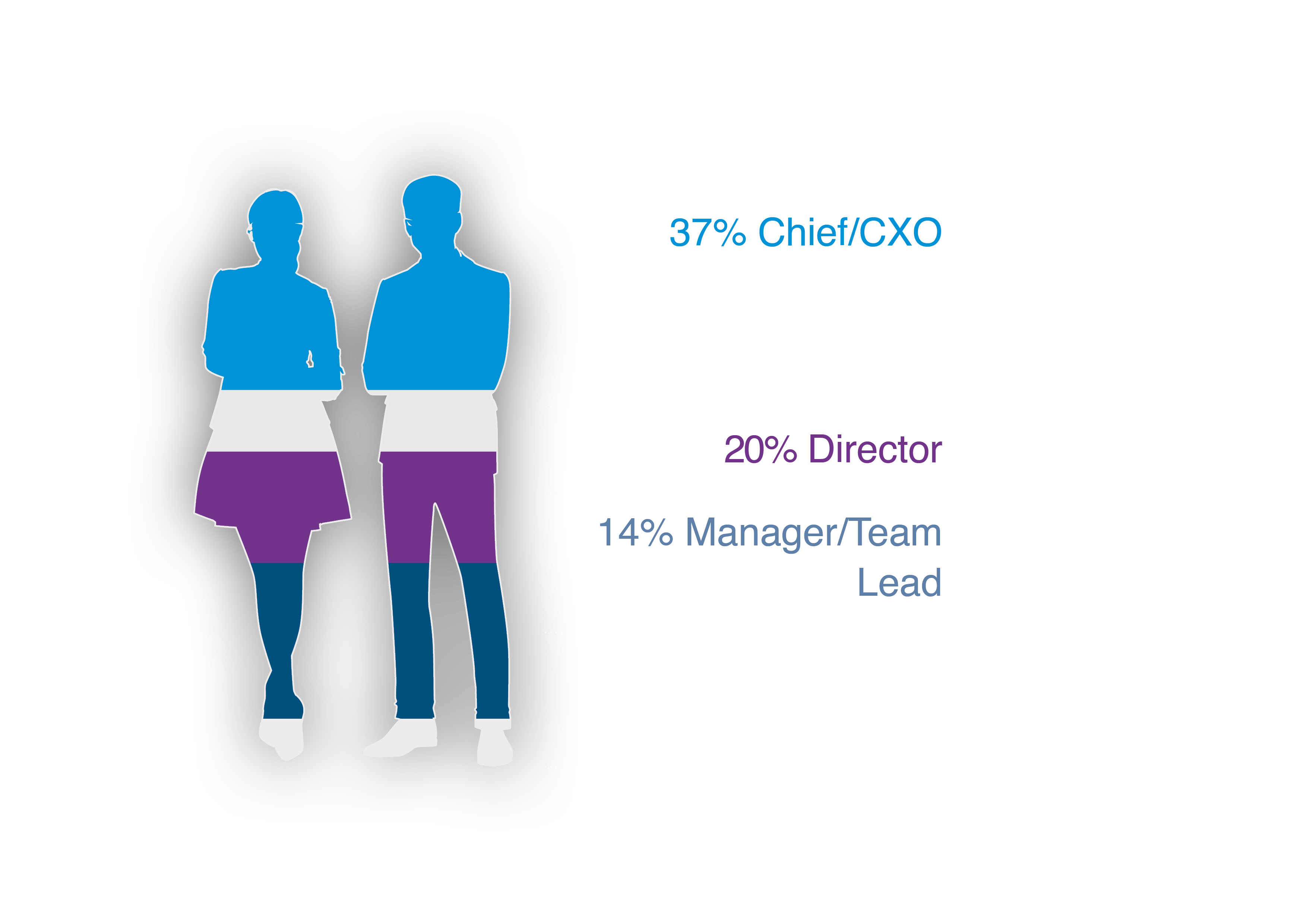
Who Will You Meet in 2024?
What Your Peers Had to Say
"It was a great networking event and there were plenty of opportunities to discuss with other colleagues attending"
Microbiotica
"The speakers were excellent and focus day was most enjoyable"
The TIM Company
